Cutting-Edge Proteomics Services
Proteomics, the large-scale study of proteins, identifies and characterizes protein functions, interactions, and modifications such as phosphorylation. By quantifying proteins across samples, proteomics reveals complex biological networks, helping to uncover disease mechanisms, discover biomarkers, and identify drug targets. It plays a crucial role in studying conditions like cancer, neurodegeneration, and metabolic disorders, as well as assessing drug effects on protein pathways.
Proteomics bridges the gap between genomics and functional biology, providing a dynamic view of protein activity. At Singulomics, we specialize in multiomics projects, providing 4D profiling and comparative data analysis across diverse sample types. By integrating ion mobility with retention time, mass-to-charge ratio (m/z), and intensity, 4D proteomics enhances traditional methods for greater resolution. From protein extraction to advanced data analysis, we deliver accurate, reproducible results with broad detection capabilities, even from low-input samples—precisely tailored to your research needs.
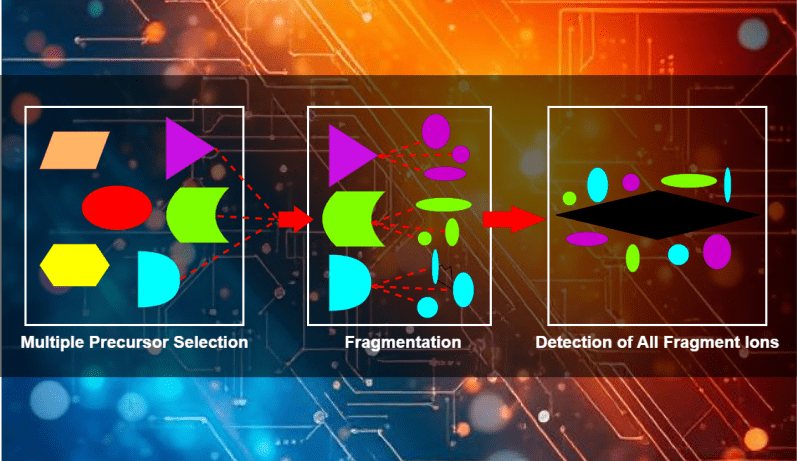
DIA Quantitative Proteomics
Data-Independent Acquisition (DIA) proteomics combines DIA mode with Parallel Accumulation–Serial Fragmentation (PASEF) technology in the diaPASEF acquisition mode, enabling comprehensive and unbiased protein quantification from complex samples. Unlike Data-Dependent Acquisition (DDA), where specific ions are selected for fragmentation, DIA acquires fragment ions for all peptides within a predefined mass-to-charge (m/z) range.
DIA proteomics provides superior sensitivity, comprehensive coverage, and accurate protein identification and quantification, even at low concentrations. Its high reproducibility across multiple runs makes it ideal for comparative and long-term studies.
Leveraging advanced DIA technology and our expert team at Singulomics, we provide accurate results in protein identification, quantification, and analysis.
Phosphoproteomics
Protein phosphorylation is a key post-translational modification that regulates protein functions, interactions, and signaling pathways within cells. Phosphoproteomics leverages liquid chromatography and mass spectrometry to efficiently identify and quantify phosphorylated proteins and peptides, precisely localize phosphorylation sites, and track changes under varying physiological conditions.
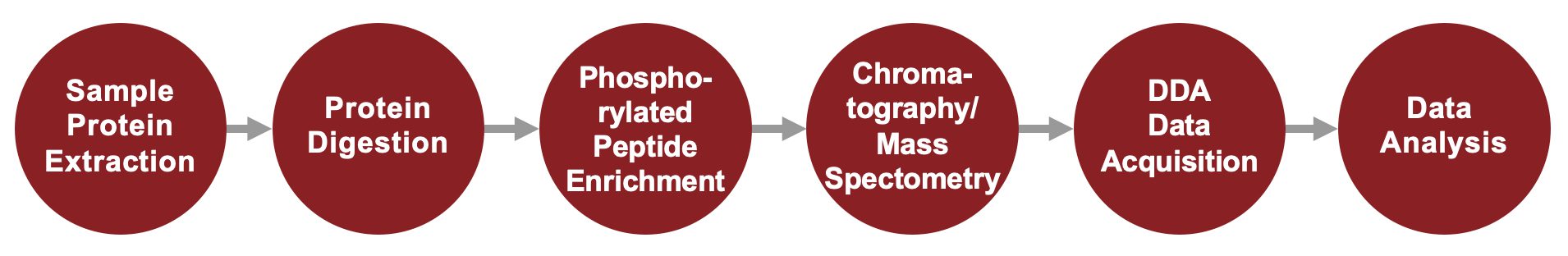
We offer peptide enrichment using phosphorylation-specific antibodies and Titanium (IV) Immobilized Metal Ion Affinity Chromatography (Ti-IMAC), combined with mass spectrometry using Data-Dependent Acquisition (DDA). After protein digestion, peptides are separated by liquid chromatography and enriched. DDA then selects the most abundant precursor ions in real time for fragmentation and sequencing, generating detailed data on phosphorylated peptide identification and quantification. By analyzing phosphorylation patterns, our approach helps decode cellular processes, uncover disease mechanisms, and identify potential therapeutic targets.
Service Workflow
With every service at Singulomics, we provide a one-stop shop complete with personalized solutions and efficient updates to help you reach your goals. We are experienced multiomics experts offering comprehensive analysis of your samples and high-quality results.
DIA Quantitative Proteomics Service Workflow
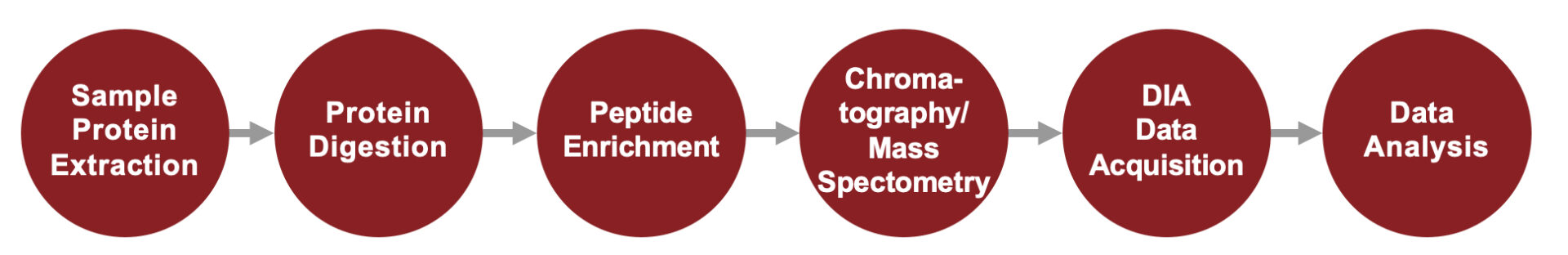
Phosphoproteomics Service Workflow
Sample Requirements
We accept a wide range of sample types for proteomics, including cells, tissues, fluids, and protein. Whether you’re working with complex clinical samples, small tissue specimens, or specific cell populations, our service is equipped to handle and process diverse sample types to meet your research needs. Please contact us to discuss the specific requirements for your sample type.
Next Steps...
Contact us to learn more or get a price quote.
